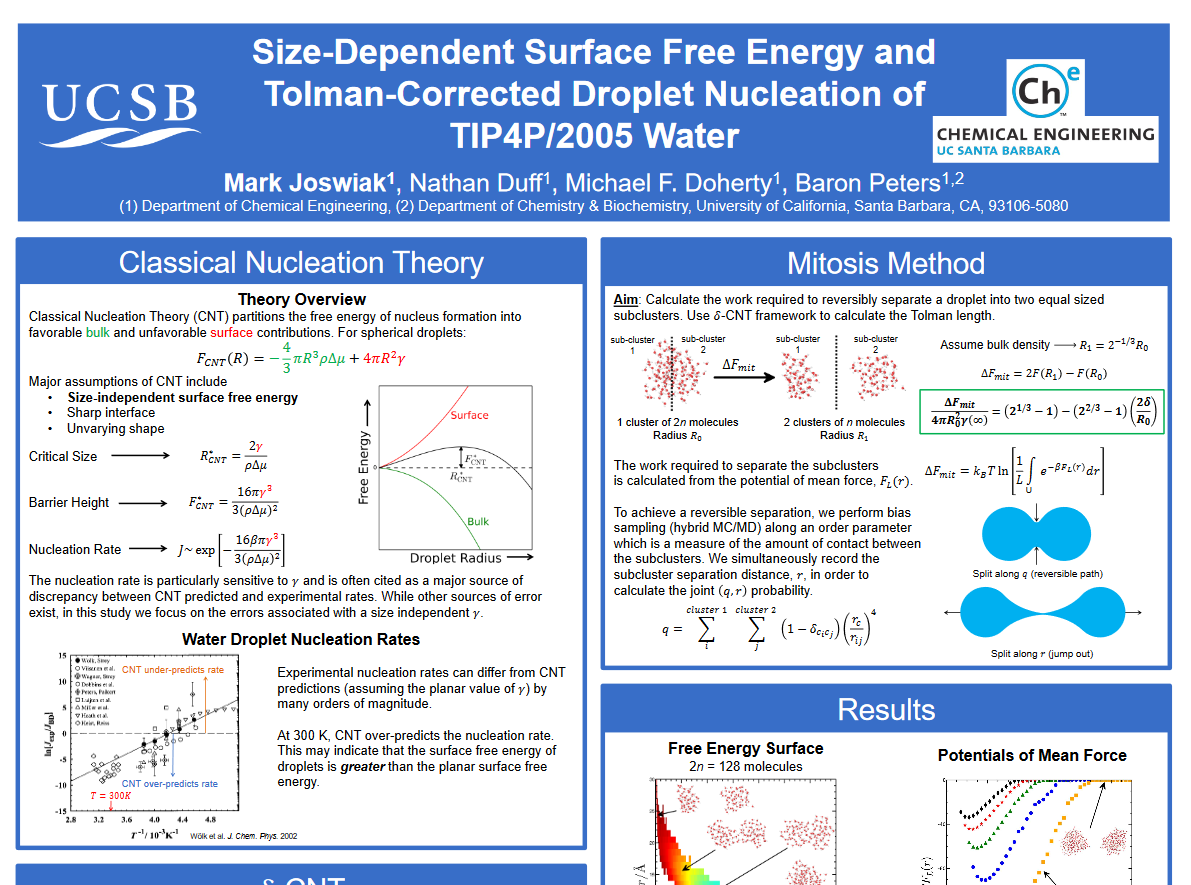
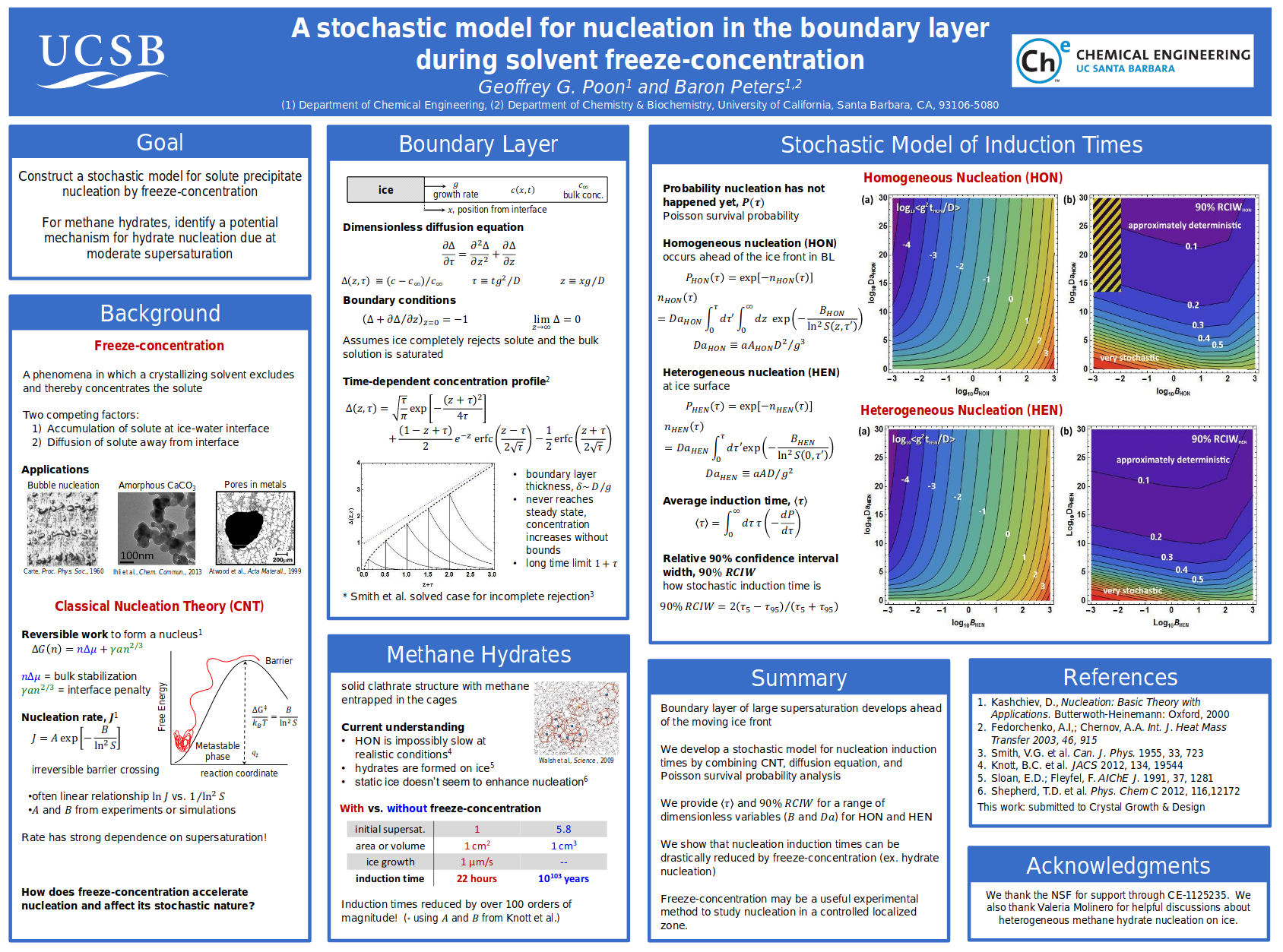
Rare Events
Activated processes like chemical reactions, nucleation, and electron transfer lead to important changes in materials and living organisms. At the molecular level these "rare events" begin as reactants, pass through transition states, and end as products in just a few fleeting moments when compared to typical lifetimes of the stable reactant and product states. Even though transition states are only briefly and rarely occupied, they are important because they determine the lifetimes of the reactants and products. These lifetimes give rise to technologically important properties like catalytic turnover frequencies and polymorph selectivity in crystallization. Because transition states are rarely and briefly occupied, they cannot be directly observed in experiments. Thus, theory and computation are valuable sources of molecular-level insight on transition states. When carefully formulated with links to observable phenomena, theory and computation provide a valuable way to test hypotheses about mechanisms and transition states at the molecular-level. For applications and examples, please see the research posters from our group below.